Over the past decades, scientists have wrestled with a problem involving the Big Bang Theory. The Big Bang Theory suggests that there should be three times as much lithium as we can observe. Why is there such a discrepancy between prediction and observation?
To get into that problem, let's back up a bit.
The Big Bang Theory (BBT) is well-supported by multiple lines of evidence and theory. It's widely accepted as the explanation for how the Universe started. Three key pieces of evidence support the BBT:
But the BBT still has some niggling questions.
The missing lithium problem is centred around the earliest stages of the Universe: from about 10 seconds to 20 minutes after the Big Bang. The Universe was super hot and it was expanding rapidly. This was the beginning of what's called the Photon Epoch.
At that time, atomic nuclei formed through nucleosynthesis. But the extreme heat that dominated the Universe prevented the nuclei from combining with electrons to form atoms. The Universe was a plasma of nuclei, electrons, and photons.
Only the lightest nuclei were formed during this time, including most of the helium in the Universe, and small amounts of other light nuclides, like deuterium and our friend lithium. For the most part, heavier elements weren't formed until stars appeared, and took on the role of nucleosynthesis.
The problem is that our understanding of the Big Bang tells us that there should be three times as much lithium as there is. The BBT gets it right when it comes to other primordial nuclei. Our observations of primordial helium and deuterium match the BBT's predictions. So far, scientists haven't been able to resolve this inconsistency.
But a new paper from researchers in China may have solved the puzzle.
One assumption in Big Bang nucleosynthesis is that all of the nuclei are in thermodynamic equilibrium, and that their velocities conform to what's called the classical Maxwell-Boltzmann distribution. But the Maxwell-Boltzmann describes what happens in what is called an ideal gas. Real gases can behave differently, and this is what the researchers propose: that nuclei in the plasma of the early photon period of the Universe behaved slightly differently than thought.
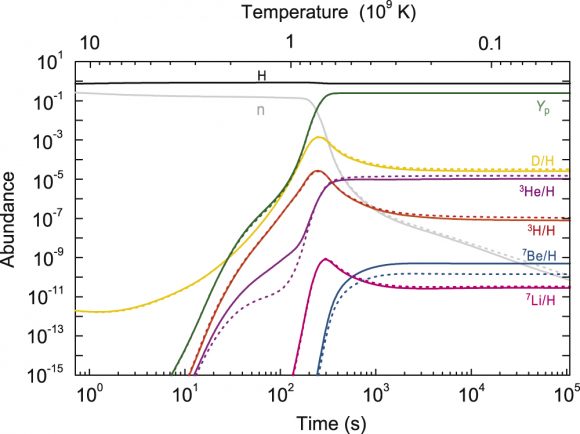
This graphics shows the distribution of early primordial light elements in the Universe by time and temperature. Temperature along the top, time along the bottom, and abundance on the side. Image: Hou et al. 2017
The authors applied what is known as non-extensive statistics to solve the problem. In the graph above, the dotted lines of the author's model predict a lower abundance of the beryllium isotope. This is key, since beryllium decays into lithium. Also key is that the resulting amount of lithium, and of the other lighter nuclei, now all conform to the amounts predicted by the Maxwell-Boltzmann distribution. It's a eureka moment for cosmology aficionados.
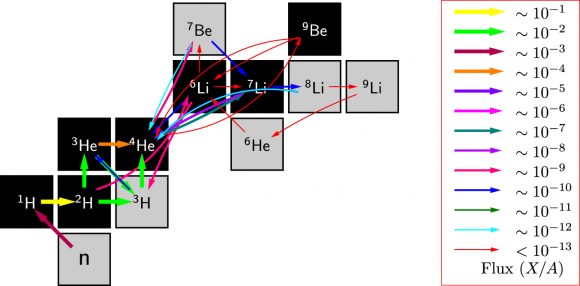
The decay chains of primordial light nuclei in the early days of the Universe. Notice the thin red arrows between Beryllium and Lithium at 10-13, the earliest time shown on this chart. Image: Chou et. al.
What this all means is scientists can now accurately predict the abundance in the primordial universe of the three primordial nuclei: helium, deuterium, and lithium. Without any discrepancy, and without any missing lithium.
This is how science grinds away at problems, and if the authors of the paper are correct, then it further validates the Big Bang Theory, and brings us one step closer to understanding how our Universe was formed.
Eureka!
No comments:
Post a Comment