For more than three decades, the internal structure and evolution of Uranus and Neptune has been a subject of debate among scientists. Given their distance from Earth and the fact that only a few robotic spacecraft have studied them directly, what goes on inside these ice giants is still something of a mystery. In lieu of direct evidence, scientists have relied on models and experiments to replicate the conditions in their interiors.
For instance, it has been theorized that within Uranus and Neptune, the extreme pressure conditions squeeze hydrogen and carbon into diamonds, which then sink down into the interior. Thanks to an experiment conducted by an international team of scientists, this "diamond rain" was recreated under laboratory conditions for the first time, giving us the first glimpse into what things could be like inside ice giants.
The study which details this experiment, titled "Formation of Diamonds in Laser-Compressed Hydrocarbons at Planetary Interior Conditions", recently appeared in the journal Nature Astronomy. Led by Dr. Dominik Kraus, a physicist from the Helmholtz-Zentrum Dresden-Rossendorf Institute of Radiation Physics, the team included members from the SLAC National Accelerator Laboratory, the Lawrence Livermore National Laboratory and UC Berkeley.
Uranus and Neptune, the Solar System's ice giant planets. Credit: Wikipedia Commons
For decades, scientists have held that the interiors of planets like Uranus and Neptune consist of solid cores surrounded by a dense concentrations of "ices". In this case, ice refers to hydrogen molecules connected to lighter elements (i.e. as carbon, oxygen and/or nitrogen) to create compounds like water and ammonia. Under extreme pressure conditions, these compounds become semi-solid, forming "slush".
And at roughly 10,000 kilometers (6214 mi) beneath the surface of these planets, the compression of hydrocarbons is thought to create diamonds. To recreate these conditions, the international team subjected a sample of polystyrene plastic to two shock waves using an intense optical laser at the Matter in Extreme Conditions (MEC) instrument, which they then paired with x-ray pulses from the SLAC's Linac Coherent Light Source (LCLS).
As Dr. Kraus, the head of a Helmholtz Young Investigator Group at HZDR, explained in an HZDR press release:
"So far, no one has been able to directly observe these sparkling showers in an experimental setting. In our experiment, we exposed a special kind of plastic – polystyrene, which also consists of a mix of carbon and hydrogen – to conditions similar to those inside Neptune or Uranus."
The plastic in this experiment simulated compounds formed from methane, a molecule that consists of one carbon atom bound to four hydrogen atoms. It is the presence of this compound that gives both Uranus and Neptune their distinct blue coloring. In the intermediate layers of these planets, it also forms hydrocarbon chains that are compressed into diamonds that could be millions of karats in weight.

The MEC hutch of SLAC's LCLS Far Experiement Hall. Credit: SLAC National Accelerator Laboratory
The optical laser the team employed created two shock waves which accurately simulated the temperature and pressure conditions at the intermediate layers of Uranus and Neptune. The first shock was smaller and slower, and was then overtaken by the stronger second shock. When they overlapped, the pressure peaked and tiny diamonds began to form. At this point, the team probed the reactions with x-ray pulses from the LCLS.
This technique, known as x-ray diffraction, allowed the team to see the small diamonds form in real-time, which was necessary since a reaction of this kind can only last for fractions of a second. As Siegfried Glenzer, a professor of photon science at SLAC and a co-author of the paper, explained:
"For this experiment, we had LCLS, the brightest X-ray source in the world. You need these intense, fast pulses of X-rays to unambiguously see the structure of these diamonds, because they are only formed in the laboratory for such a very short time."
In the end, the research team found that nearly every carbon atom in the original plastic sample was incorporated into small diamond structures. While they measured just a few nanometers in diameter, the team predicts that on Uranus and Neptune, the diamonds would be much larger. Over time, they speculate that these could sink into the planets' atmospheres and form a layer of diamond around the core.
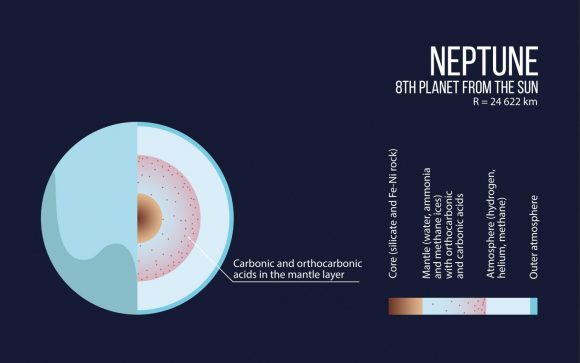
The interior structure of Neptune. Credit: Moscow Institute of Physics and Technology
In previous studies, attempts to recreate the conditions in Uranus and Neptune's interior met with limited success. While they showed results that indicated the formation of graphite and diamonds, the teams conducting them could not capture the measurements in real-time. As noted, the extreme temperatures and pressures that exist within gas/ice giants can only be simulated in a laboratory for very short periods of time.
However, thanks to LCLS - which creates X-ray pulses a billion times brighter than previous instruments and fires them at a rate of about 120 pulses per second (each one lasting just quadrillionths of a second) - the science team was able to directly measure the chemical reaction for the first time. In the end, these results are of particular significance to planetary scientists who specialize in the study of how planets form and evolve.
As Kraus explained, it could cause to rethink the relationship between a planet's mass and its radius, and lead to new models of planet classification:
"With planets, the relationship between mass and radius can tell scientists quite a bit about the chemistry. And the chemistry that happens in the interior can provide additional information about some of the defining features of the planet... We can't go inside the planets and look at them, so these laboratory experiments complement satellite and telescope observations."
This experiment also opens new possibilities for matter compression and the creation of synthetic materials. Nanodiamonds currently have many commercial applications - i.e. medicine, electronics, scientific equipment, etc, - and creating them with lasers would be far more cost-effective and safe than current methods (which involve explosives).
Fusion research, which also relies on creating extreme pressure and temperature conditions to generate abundant energy, could also benefit from this experiment. On top of that, the results of this study offer a tantalizing hint at what the cores of massive planets look like. In addition to being composed of silicate rock and metals, ice giants may also have a diamond layer at their core-mantle boundary.
Assuming we can create probes of sufficiently strong super-materials someday, wouldn't that be worth looking into?
Further Reading: SLAC, HZDR, Nature Astronomy
No comments:
Post a Comment